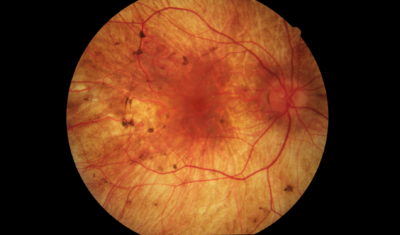
Choroideremia Research Is Necessary to Help Prevent Blindness at an Early Age
Choroideremia is an X-linked, recessive, inherited retinal disorder that affects 1 in 50,000 individuals. X-linked refers to the X chromosome, which is one of the chromosomes that determines gender. Females receive an X chromosome from each parent; therefore, females can be carriers of the disease and asymptomatic if choroidermemia is passed down from only one parent. Because males receive one X chromosome from their mother and one Y chromosome from their father, they will fully express the symptoms of the disease if it is passed down through the X chromosome, making it significantly more common for the disorder to affect males rather than females.
Symptoms of choroideremia may begin as night blindness and peripheral vision loss in adolescence and may result in the reduction of central vision as the years go by. Legal blindness from peripheral vision loss can happen as early as 30 to 40 years of age, and severe central vision loss can be seen by the time one is 50 to 70 years of age.
Choroideremia can be difficult to diagnose correctly, especially in early stages of the disease. It is often confused with other retinal degeneration conditions, such as retinitis pigmentosa. The most conclusive way to diagnose a patient with choroideremia is by having DNA analyzed by a certified laboratory.
The Retina Foundation, located in Dallas, Texas, is at the forefront in facilitating this type of testing. We evaluate the visual function of the patient, send the DNA sample to a certified lab for mutation screening, and advise the patient when the results come back. In addition, the Retina Foundation currently has several ongoing clinical research studies for choroideremia. These include both natural history studies and gene therapy clinical trials.
Natural history studies are vital when designing clinical trials because they give insight to the rate at which a disease progresses and provide researchers the necessary information to establish the different stages of a disease. It is because of natural history studies that researchers across the world are able to collaborate and discuss choroideremia and the disorder’s typical visual function decline over time. These studies are also important for identifying sensitive outcome measures. These are measures such as visual acuity or visual fields, which worsen as the disease progresses. Knowing which measures are most sensitive to change is critical for designing a clinical trial.
The cause of choroideremia is a genetic mutation (mistake in the DNA code) in the gene called CHM. This gene makes an escort protein (REP-1) that is vital to cell function throughout the body, but especially in the eye. A mutation can cause the wrong protein to be made, or make no protein at all. A gene therapy clinical trial aims to provide the correct REP-1 protein through an injection of the normal gene into the eye. The protein is introduced into the eye with the aid of a harmless virus called AAV2. The virus has been altered to hold the REP-1 protein and, hopefully, should repair the cells in the retina. There is also the possibility that it can rescue the surviving cells and prevent future vision loss.
Those experiencing the effects of choroideremia will gradually lose the ability to drive and walk without assistance. Finding employment opportunities that will accommodate their needs may become difficult and result in economic instability. As the disease progresses with age, those diagnosed will find that they are unable to recognize the faces of their loved ones, such as their children and grandchildren. Therefore, those diagnosed with choroideremia can experience a loss in independence, as well as quality of life. The goal for the choroideremia research conducted at the Retina Foundation is to discover safe treatment options and to provide hope for those living with the disease so they can live life to the fullest.