Inherited Retinal Degeneration
Clinic and Laboratory

Mark Pennesi, MD, PhD, FARVO, joined the Retina Foundation in 2024 as Director of the Inherited Retinal Degeneration Clinic and Laboratory.

Advancements
- Established the IRD Clinic with oversight of more than 1,000 patients from across the globe.
- Quickly launched new IRD clinical trials – currently there are 25 active trials including several gene therapy trials for Retinitis Pigmentosa (RP) and Stargardt disease.
- Helped grow clinical trial revenue by $1 million.
- Secured joint grant funding with the Casey Eye Institute for the next generation of high-resolution OCT imaging and deep machine learning for image segmentation.
- Launched stem cell clinical trial for RP with the potential to recover vision that has already been lost.
- Secured funding from Crystal Charity Ball to establish a Pediatric IRD Clinic with specialized equipment to care for the children.
- Continued gene therapy trial for X-linked RP, a severe form of the disease that makes up 15% of those with RP and Stargardt disease, the most common pediatric macular dystrophy.
Clinical Trials
Please contact us at 214-363-3911 if you are interested in being a part of our clinical studies or an appointment with Dr. Mark Pennesi.
Experts
Mark Pennesi, MD, PhD, FARVO
Kaylie Jones
Sr. Research Associate
Martin Klein
Lab Manager
Jennifer Herrera
Clinical Coordinator
Destiny Coleman
Ophthalmology Technician
Grant Koenigsman
Ophthalmology Technician
Rachel Yoken
Ophthalmic Technician
Rachel Murray
Clinical Research Coordinator
Overview:
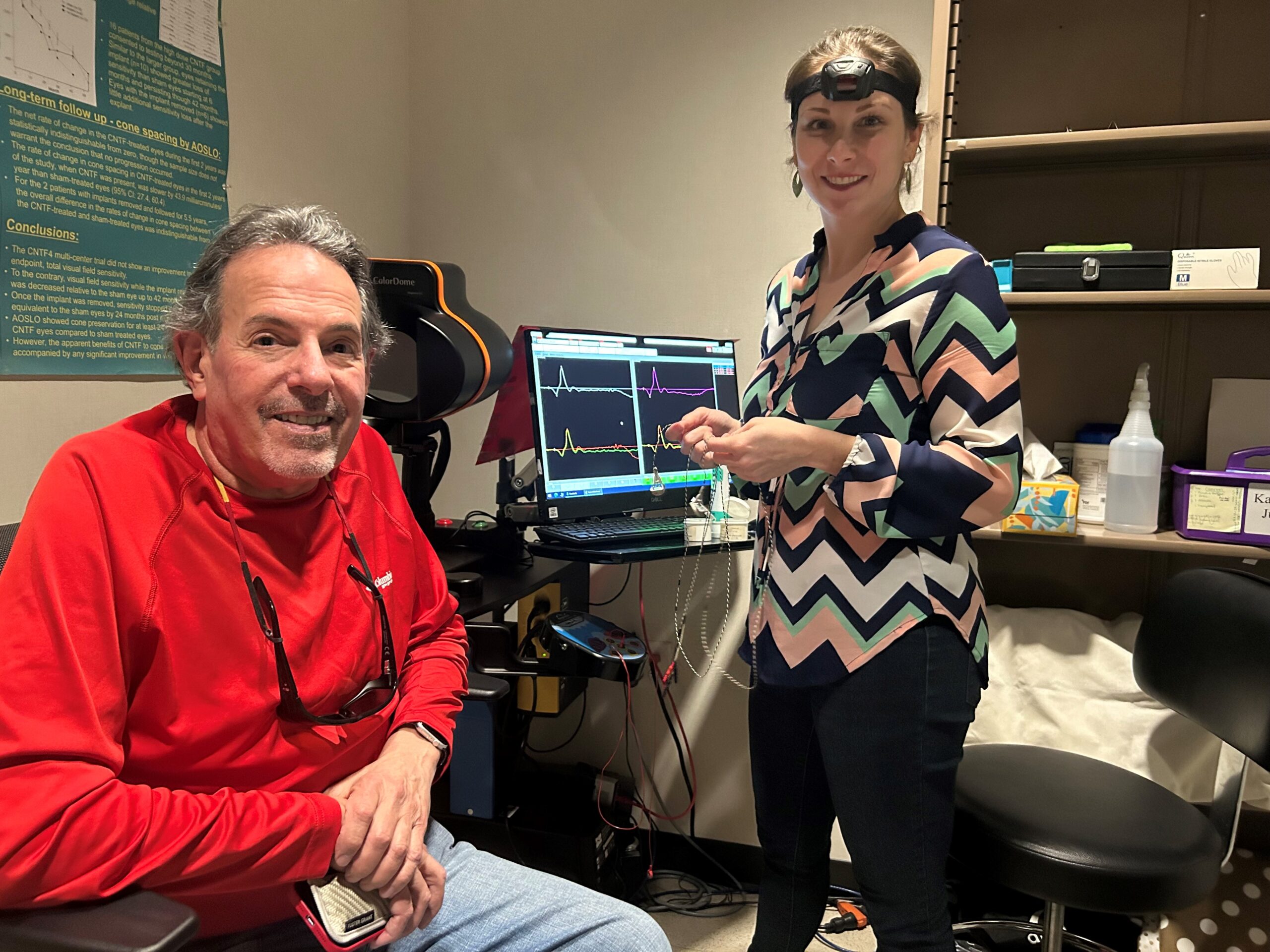
Led by Dr. David Birch, PhD, The Rose-Silverthorne Retinal Degenerations Laboratory collaborates with global research partners to identify causes, treatments and cures for inherited eye diseases. Patients visit the lab for genetic testing and state-of-the-art assessments for visual function. The lab also conducts numerous clinical trials to determine the safety and efficacy of potential treatments for individuals with inherited eye diseases.
Advancements
Established a model for deriving photoreceptor properties from the a-wave of the electroretinogram. This has been used in many labs to understand disease processes in retinitis pigmentosa and allied retinal degenerations.
Helped create and characterize the first model of Stargardt disease, the abca4 (abcr) knockout mouse.
Established the Southwest Eye Registry for patients with inherited retinal diseases and allied disorders.
Worked with neonatologists to identify DHA as an essential component of milk for low birthweight infants.
Led early development of the Argus II Retinal Prosthesis System to help patients who are blind due to advanced retinitis pigmentosa. When using this “bionic eye” artificial retina, patients can distinguish letters, objects, trees and much more.
Helped identify several genes associated with retinitis pigmentosa, including a new gene responsible for retinitis pigmentosa in several Hispanic families in Texas.
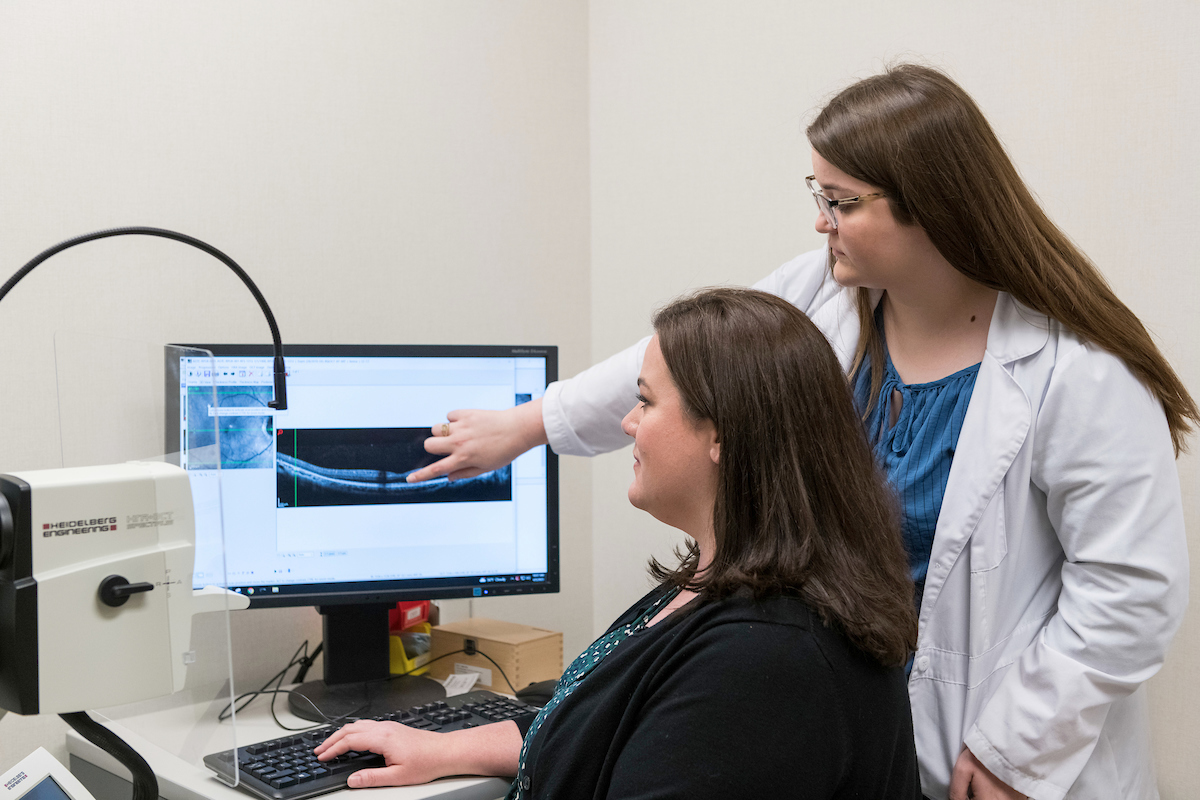
Established the “ellipsoid zone” as a structural measure of photoreceptor integrity. This is now being used as an outcome measure in numerous clinical trials for Inherited Retinal Degenerations.
Lead center for determining whether RPGR replacement gene therapy is safe and efficacious in x-linked retinitis pigmentosa.
Published 400 peer-reviewed articles on research findings.
Clinical Trials
Please contact Kirsten Locke at 214-363-3911 if you are interested in being a part of our clinical studies.
Experts
David Birch, PhD
Scientific Director
Kirsten Locke, CRA, FOPS
Clinical Trials Manager
Martin Klein, MS
Lab Manager
Kaylie Jones, MS
Senior Research Associate
Eric Vasquez
Clinical Research Coordinator
Jennifer Herrera
Ophthalmic Technician
Get expert help
Get the Latest Updates
Sign up to receive the “Recently @Retina” newsletter to keep up-to-date.